Background: Allogeneic hematopoietic cell transplantation (alloHCT) is the approach that offers the highest curative rate for acute myelogenous leukemia (AML) with intermediate or high-risk cytogenetics. Graft versus host disease (GvHD) has remained the main cause of post-transplantation mortality and morbidity, despite advances in prophylaxis and therapy, adding significant economic burden and affecting quality of life. It would be desirable to reduce the rate of GvHD among patients in complete remission (CR) without increasing the risk of relapse. In this study, we have developed a novel conditioning regimen of total marrow and lymphoid irradiation (TMLI) at 2000 cGy, together with post-transplant cyclophosphamide (PTCy), to 1) reduce the possibly increased risk of relapse from PTCy, by using escalated radiation doses of TMLI, as increasing radiation doses has the potential to decrease the post-transplantation relapse rate (Blood, vol 76, pp. 1867-1871, 1990); and 2) reduce the risk of chronic GvHD by using PTCy. The major goal of this pilot study of TMLI and PTCy (clinicaltrials.gov: NCT03467386), was to thus improve GvHD-free/relapse-free survival (GRFS), reported to be 45% from total body irradiation (TBI) and tacrolimus/sirolimus prophylaxis (BBMT, vol 26(2), pp. 292-299, 2020), in patients with AML in remission.
Patients and Methods: A total of 18 patients were enrolled and treated (see Table) between March 2018 and December 2019. Key criteria were ages 18 to 60, first or second CR, minimal residual disease negative by multi-color flow cytometry, and normal organ function. TMLI was administered on days -4 to 0 without addition of chemotherapy. The radiation dose for all patients (n=18) was 2000 cGy, delivered in 200 cGy fractions twice daily. The radiation dose delivered to the liver and brain was kept at 1200 cGy. Remaining organs were considered non-targeted. All patients received peripheral blood stem cells on day 0. Cyclophosphamide was given on days +3 and +4, 50 mg/kg each day for GVHD prevention. Tacrolimus, 1 mg continuous infusion adjusted to maintain levels from 5 to 10 ng/mL was given from day +5 to day +90, and G-CSF 5 µg/kg daily was administered at day +5 until recovery of neutrophil counts.
Endpoints included toxicity, GRFS at 1 year, engraftment, overall survival (OS), and non-relapse mortality (NRM). Toxicities were defined according to the Bearman and CTCAE 4.03 scales, the latter for hematologic toxicity. A patient safety lead-in segment (n=6) was conducted to ensure that there were no unexpected toxicities, allowing for a dose de-escalation to 1800 cGy. GRFS was defined as grade 3-4 acute GvHD, chronic GvHD requiring systemic treatment, relapse, or death (from any cause), whichever occurred first.
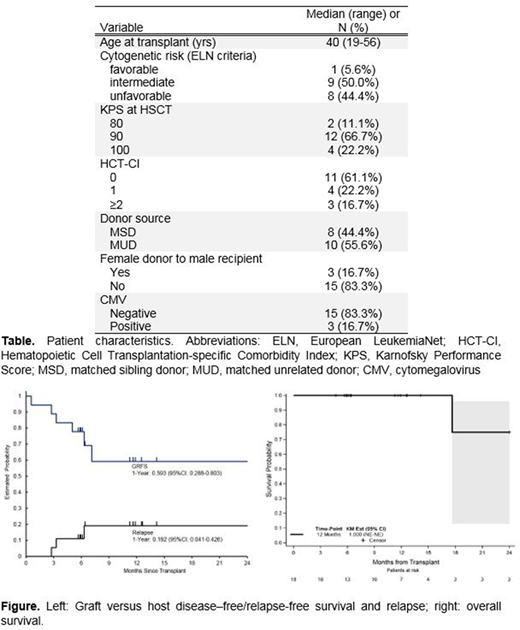
Results: Bearman toxicity data are available for all patients. Among these patients, grade 2 toxicities were bladder toxicity and stomatitis. No grade 3-4 toxicities or toxicity-related deaths were observed. Acute GVHD (aGVHD) developed in 2 of patients (100-day Grade II-IV aGVHD: 11.1%, 95%CI: 1.7-30.4); of those, only 1 patient developed Grades III-IV (100-day Grade III-IV aGVHD: 5.6%, 95%CI: 0.3-23.1). Five patients developed mild chronic GVHD (1-year cGVHD rate: 28.6%, 95%CI: 7.5%-54.7%). The GRFS rate at 1 year was 59.3% (95% CI: 28.8-80.3) (Figure).
The median follow up was 11.3 months (range 4.7 to 25.4) for surviving patients (n=17). All patients engrafted. Time to neutrophil and platelet recovery were 14 days (range 13-32 days) and 20 days (range 11-49 days), respectively. One-year estimates of OS and relapse-free survival were 100% and 80.8% (95% CI: 50.5-93.6), respectively (Figure). Disease relapse at 1 year was 19.2% (95% CI: 4.1-42.6). The estimates of NRM at 100 days and 1 year were both 0%. Relapsed disease after transplant occurred in 3 patients (16.7%). One patient died after relapse.
Conclusions: 1) This chemotherapy-free conditioning regimen, together with PTCy and tacrolimus, is safe and feasible, with no NRM. 2) All patients achieved engraftment. 3) Participants with ≥1 year follow-up have discontinued immunosuppressive therapy, reducing financial burden and leading to improved quality of life. The preliminary results suggest an improved GRFS rate. A larger phase 2 trial is in preparation to corroborate these data.
Stein:Amgen: Consultancy, Speakers Bureau; Stemline: Consultancy, Speakers Bureau. Al Malki:Neximmune: Consultancy; Jazz Pharmacuticals, Inc: Consultancy; Rigel Pharma: Consultancy. Ali:Incyte Corporation: Consultancy. Aribi:Seattle Genetics: Consultancy. Marcucci:Novartis: Speakers Bureau; Takeda: Other: Research Support (Investigation Initiated Clinical Trial); Pfizer: Other: Research Support (Investigation Initiated Clinical Trial); Abbvie: Speakers Bureau; Merck: Other: Research Support (Investigation Initiated Clinical Trial); Iaso Bio: Membership on an entity's Board of Directors or advisory committees. Nakamura:Alexion: Other: Support on a meeting presentation; Kyowa-Kirin: Other: Support on a meeting presentation; Merck: Other: advisory board meeting; Celgene: Other: Support on seminar; Magenta Therapeutics: Other: Advisory board meeting; Viracor: Consultancy; Kadmon Corporation: Other: Advisory board meeting; NapaJen Pharma: Consultancy. Pullarkat:AbbVie, Inc.: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genetech: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dova: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salhotra:Celgene: Research Funding; Kadmon: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal